Ethane Molecular Structure and Orbital Interaction Diagram.
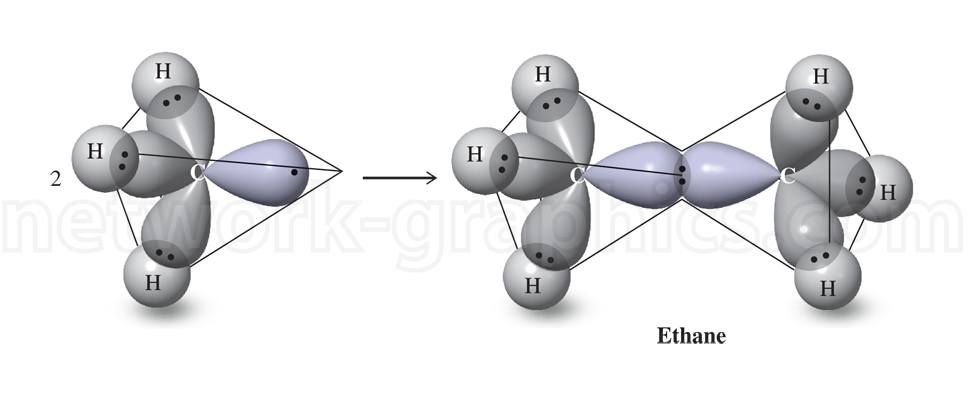
This illustration depicts the molecular structure and orbital interaction in ethane (C₂H₆). It highlights the formation of sigma (σ) bonds between the carbon atoms via the overlap of sp³ hybridized orbitals. Each carbon atom forms bonds with hydrogen atoms and one sigma bond between the two carbon atoms. The diagram effectively shows the bonding geometry and orbital interactions that create the molecular structure of ethane, a basic alkane in organic chemistry.
This image is ideal for chemistry textbooks focusing on organic chemistry, chemical bonding, and molecular orbital theory, offering students a clear visual representation of orbital hybridization and the tetrahedral geometry around carbon atoms.
We can provide sample images or create custom illustrations tailored to your projects. If you are looking for an illustration of this type, or from another subject area, you can contact us to discuss your needs.
Network Graphics / Division of Abramson & Wickham Graphics Inc.
All rights reserved.

